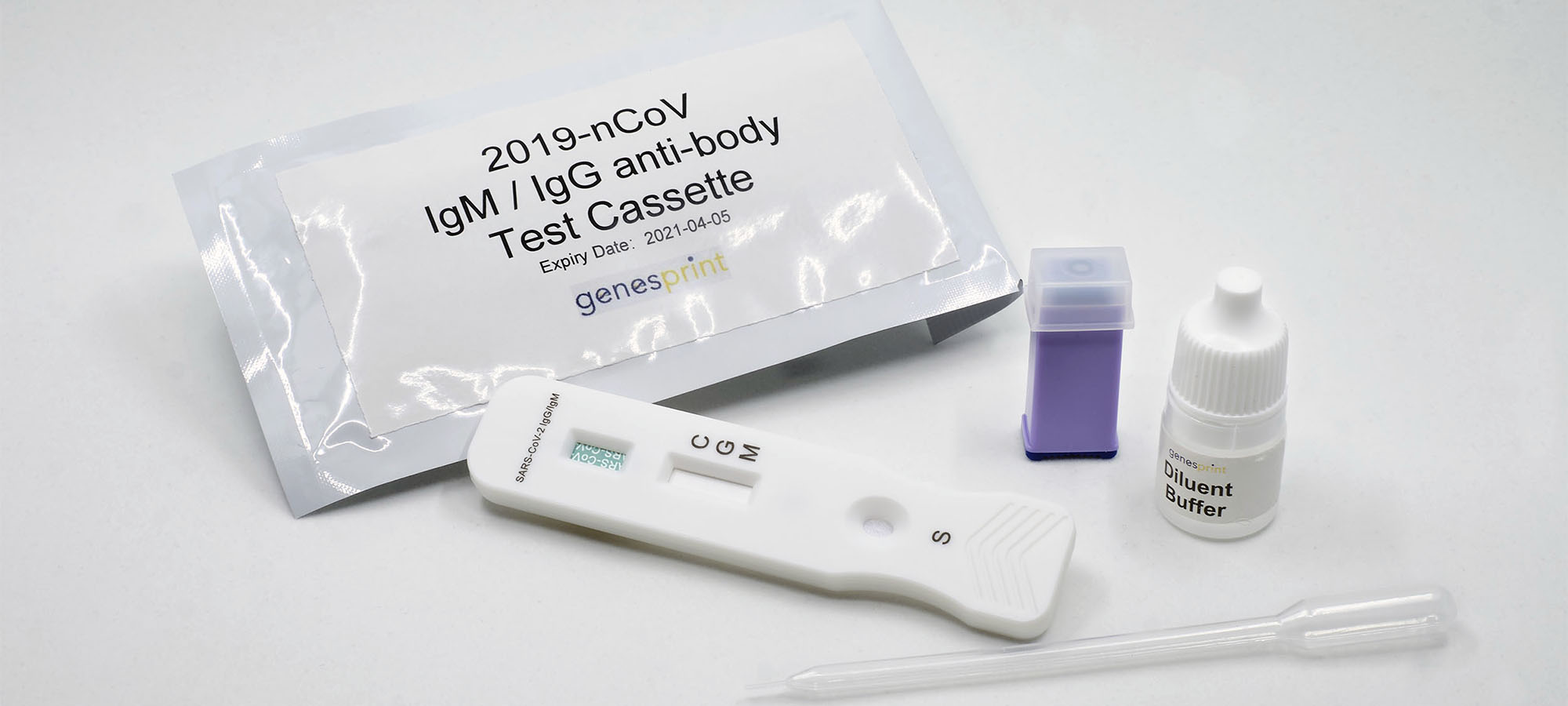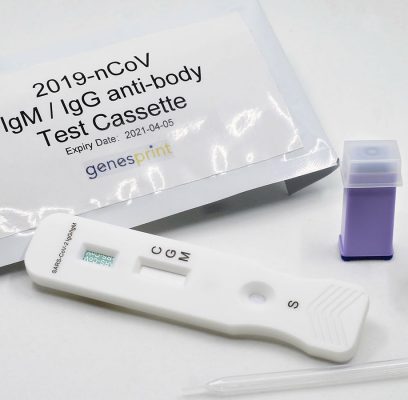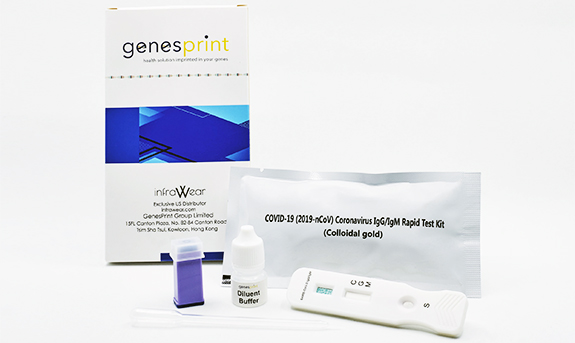
Genesprint Rapid IgM-IgG Combined Antibody Test for COVID-19 is used to qualitatively detect IgG and IgM antibodies of the novel coronavirus in human serum, plasma, or whole blood in vitro.
✓ Tests for early stage (IgM) and later stage (IgG) antibodies
✓ 20 minute detection period
✓ Simple test procedure
✓ Easy to read results
✓ Only 2 drops of blood required
✓ 98.74% specificity
✓ No lab required
✓ Not for sale in the US

InfraWear, LLC is the exclusive US distributor for the GenesPrint COVID-19 IgM/IgG Rapid Test Kit – Finger prick test
PREPARATION
ROOM TEMPERATURE: Open the package, remove all contents, place them at room temperature, and wait for 30 minutes.
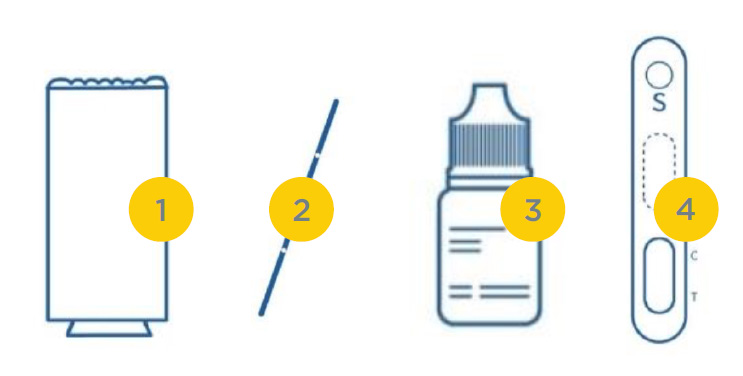
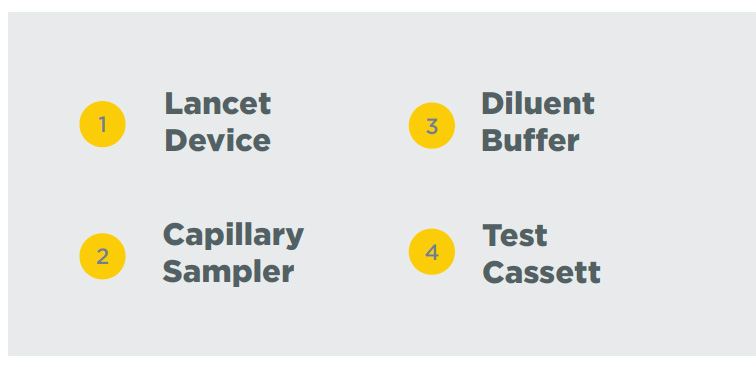
SAMPLING
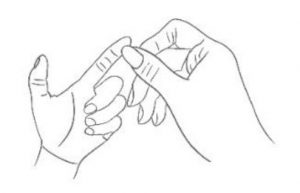
1. Massage Massage your left index finger in order to have it naturally filled with blood. Don’t massage too hard.
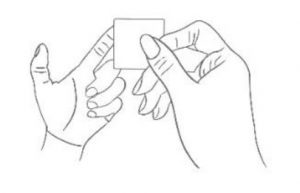
2. Sterilization Sterilize your fingertip with alcohol swab.
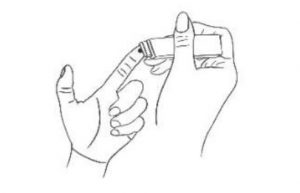
3. Pricking Hold the lancet device with right hand, prick the fingertip with the lancet device to a depth of 2-3mm.
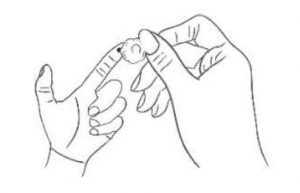
4. Swab Wipe the first drop of blood with cotton ball.
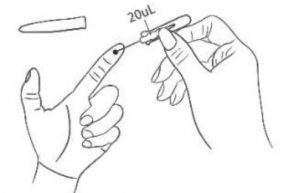
5. Sampling Collect blood specimen to 20μL tick mark using the capillary sampler; if the blood flow is not smooth, apply pressure to the left index fingertip using your right hand. After sufficient specimen is collected, cover the puncture site with the cotton ball to stop bleeding.
TEST PROCEDURE
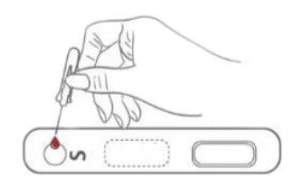
1. Testing Dispense 20μL blood specimen into the Test Cassette sample well.
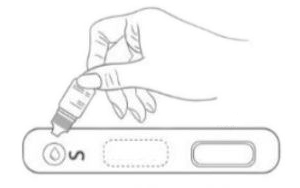
2. Diluent Dispense 2-3 drops of diluent buffer into the Test Cassette sample well. Start the timer.
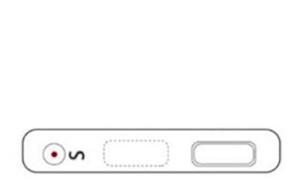
3. Result Allow test to run for 10 to 20 minutes. Read the results by viewing the detection window. Test results that have run over 20 minutes are invalid.
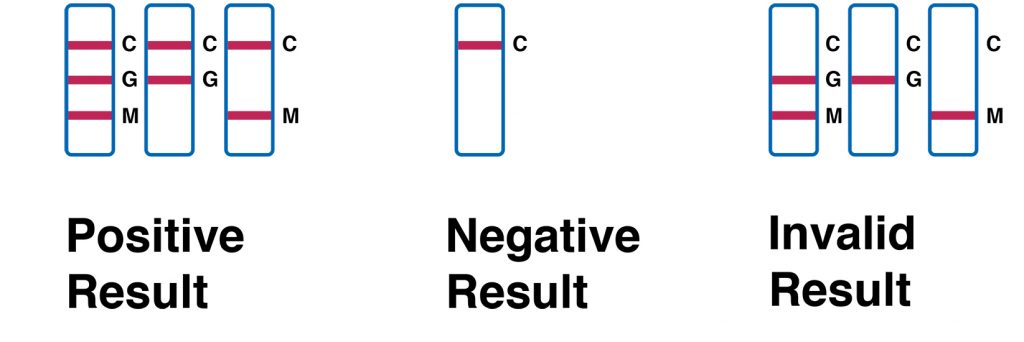
INTERPRETATION
For more information or to order: info@infrawear.com
Click here for more details on the COVID-19 IgM/IgG Rapid Test Kit
- This test has not been reviewed by the FDA.
- Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus. Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals.
- Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
- Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
- Not for the screening of donated blood.